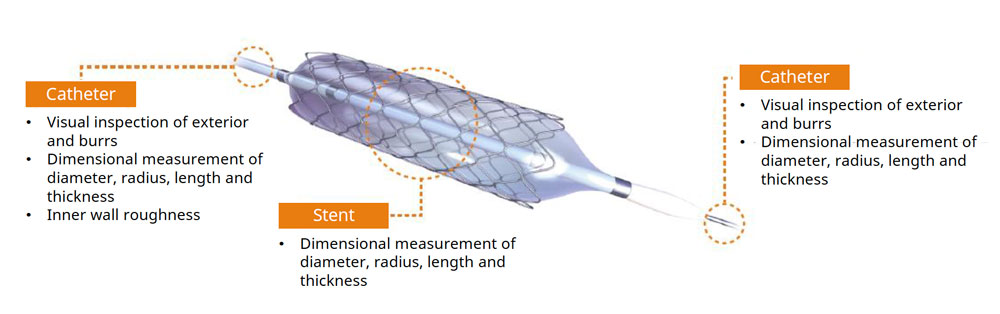
The medical device manufacturing industry continues to grow rapidly due to increased global demand. Reasons for this growth include an aging population, a rise in lifestyle-related diseases, the implementation of medical insurance systems in developing countries, and globalization. Medical devices must be easy to use, reliable, and durable to protect the lives of patients and ensure their safety.
Medical device manufacturers build and maintain effective quality management systems that comply with strict international laws and regulations. At some medical equipment manufacturing sites, employees perform complicated manual work using a microscope for each item in high-mix, low-volume production. Maintaining the health and safety of these employees during long working hours is a major concern. Other top challenges include reducing human error in operations and improving the efficiency of technical training for workers.
Our solutions contribute to the development of medical device manufacturing sites with improved work efficiency, training, and quality, as well as safer and more comfortable operation of optical microscopes.
Our Solutions
Quality Management
| Safety, Health Maintenance, and Operational Efficiency |
Fast and
| Measurement and Inspection Case Studies |
Solutions for Quality Management and Regulation
Medical devices can impact human health and safety, so safe and effective products and services are mandatory and strictly regulated. Medical device manufacturers build reliable quality management systems to consistently meet customer and regulatory requirements.
Solutions used in the implementation of a manufacturers’ quality management system should be based on the international standard ISO13485:2016, which is specialized for medical devices, and should comply with the regulatory requirements of each country, including Title 21 CFR Part820 QSR in the United States.
Our Solutions
We provide the following product and service solutions for maintenance during and after installation, enabling compliance with ISO13485:2016 as well as your country’s specific laws and regulations.
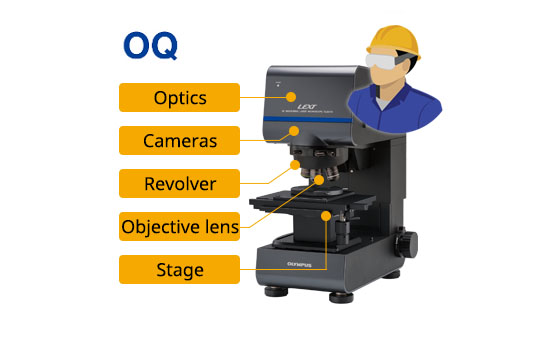
Installation qualification (IQ) operational qualification (OQ) process: | ||
IQ: Technician installs the system and confirms if installed correctly |
OQ: Confirms if the system operates correctly in the installation environment |
Provides IQ/OQ documents |
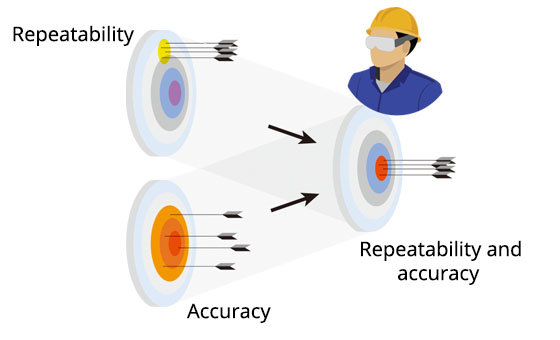
Calibration: | ||
Confirms accuracy and repeatability in the installation environment |
Issues calibration certificate |
After installation, our service technicians can perform OQ, provide OQ documents, and return to calibrate the system at regular intervals.
Recommended Microscopes for Quality Management and Regulation
DSX1000Digital Microscopes
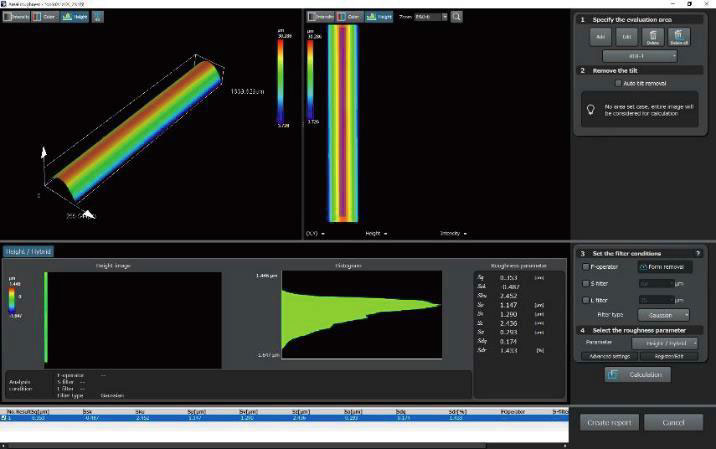
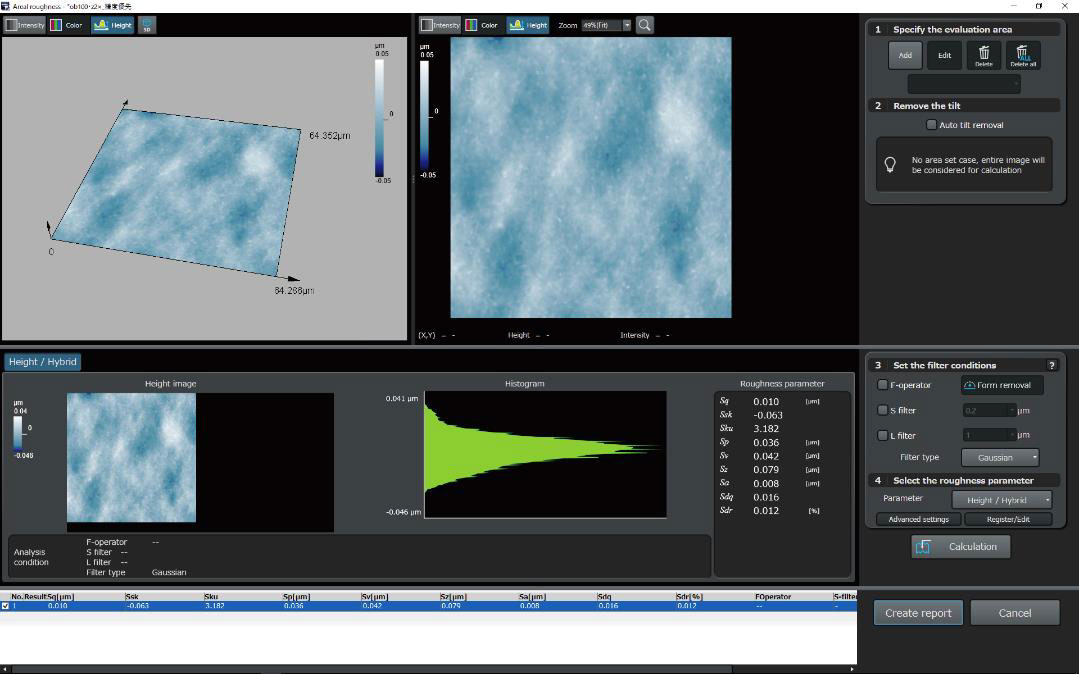
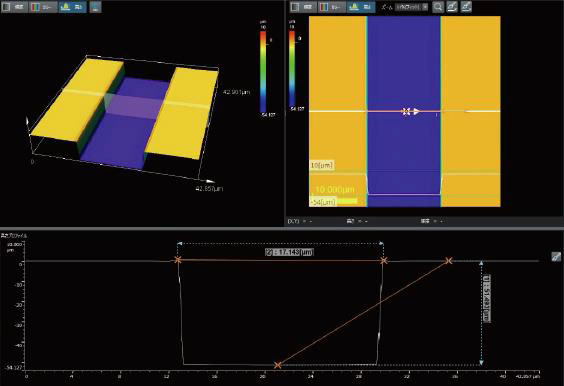
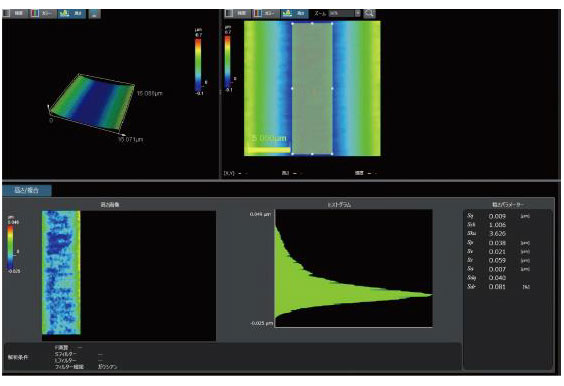
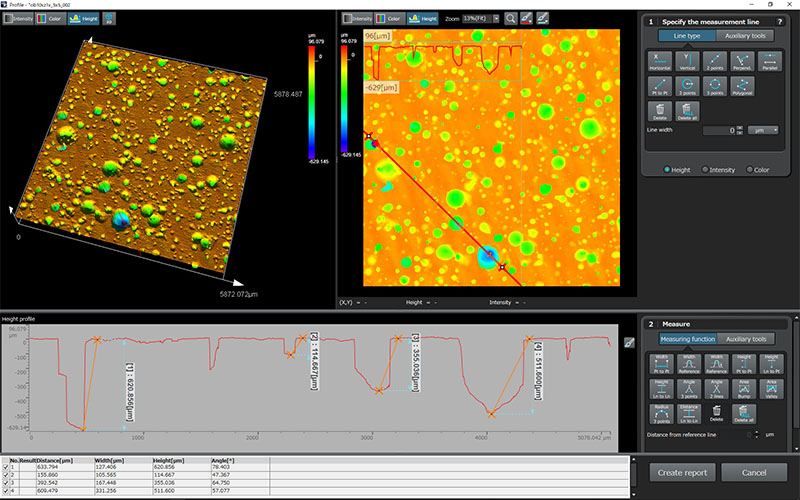
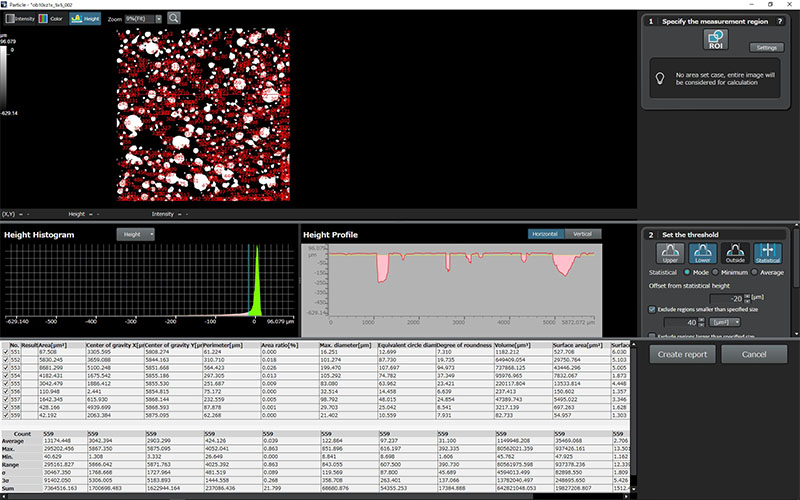
| OLS51003D Laser Confocal Microscopes
|
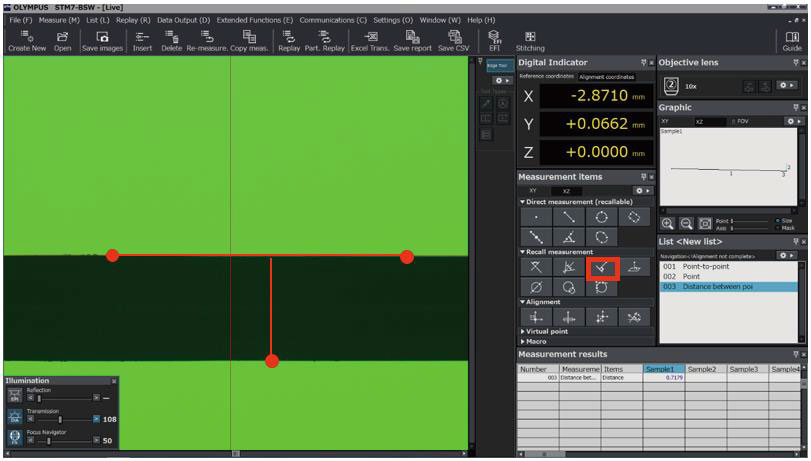
STM7Measuring Microscopes
| CIX100Cleanliness Inspector
*Supported in selected countries only. |
Ergonomic Solutions for Efficiency and Occupational Health and Safety
In the assembly process of precision parts in medical equipment manufacturing, the operator may use a microscope for long durations, making ergonomics critical for user comfort. If forced to continue to work for long periods with non-ergonomic equipment, operators may experience health problems and injuries.
Our SZX™ series (SZX7, SZX10, SZX16) stereo microscopes are ergonomically designed to help users work comfortably and stay safer and healthier. To enable each operator to work in a comfortable position, the microscope’s parts and functions can be personalized and adjusted, for example, to the height of each new user.
Solutions for Improved Microscope Ergonomics
SZX7/SZX10/SZX16Stereo Microscopes
| Blog Post
The Importance of Integrating Ergonomics
|
Stereo Microscope Ergonomics |
Solutions for a Faster, More Efficient Assembly Process
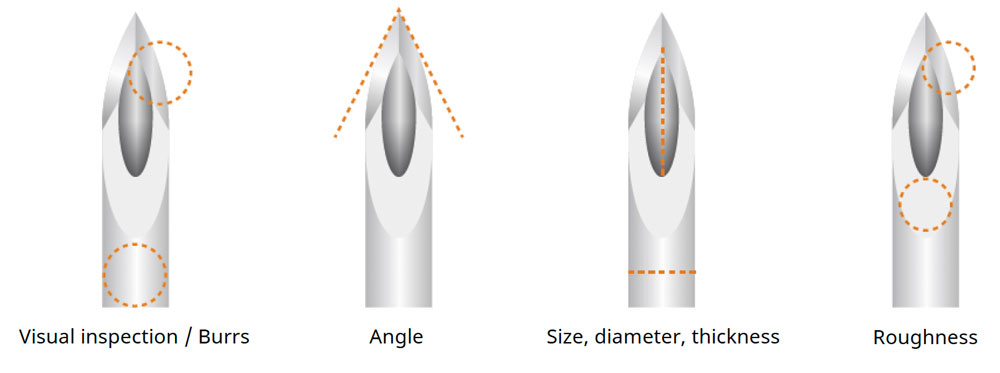
Due to unique and complex product characteristics, medical devices are often assembled and inspected manually using microscopes. The products produced include a wide variety of devices in small quantities, so operators often need to switch work procedures each time a new item is placed under the microscope. The frequent switching of procedures can lead to errors during assembly, as operators must repeatedly look away from the eyepieces to check the instructions.
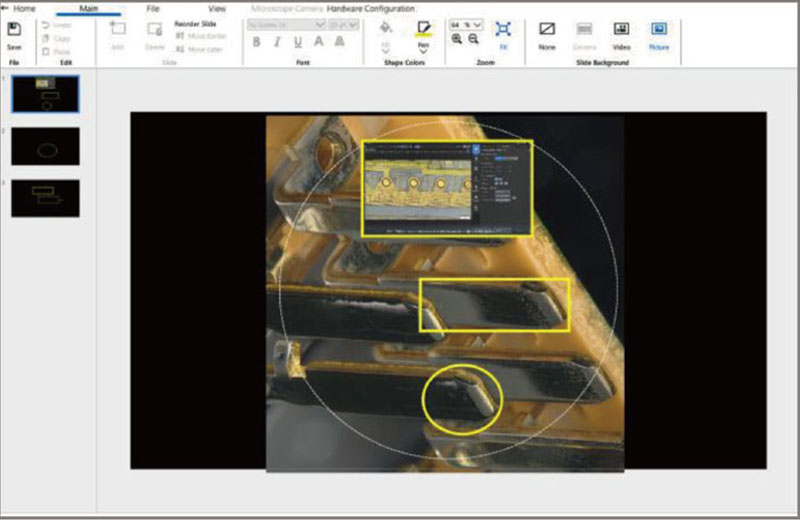
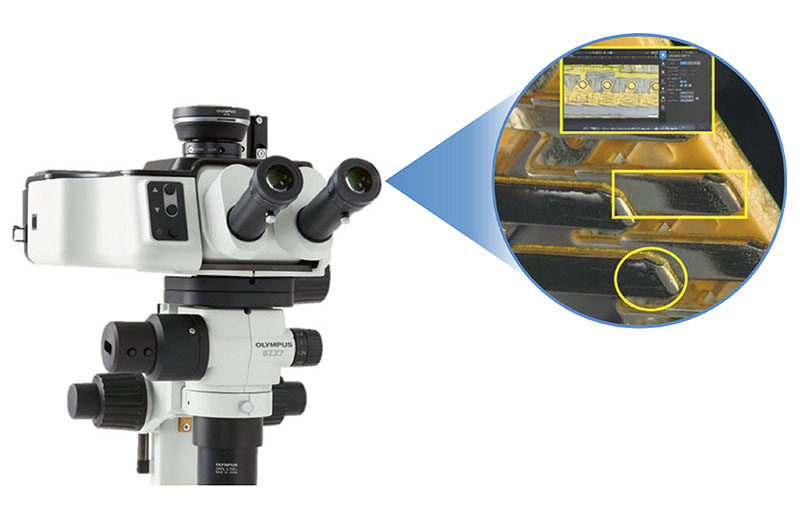
Our SZX-AR1 augmented reality microscope system overcomes these challenges by projecting work procedures as digital information into the eyepieces of your stereo microscope. The digital projection of text, digital images, and videos helps improve the speed and quality of medical device assembly and inspection. The system can also be used with third-party collaboration software to streamline training and problem-solving with remote guidance.
SZX-AR1 software |
Using the SZX-AR1 software, work instructions, videos, images, and annotations can be displayed in the microscope’s field of view. |
Augmented Reality Microscope Solutions for Work Efficiency
SZX-AR1AR Microscope
The SZX SZX-AR1 augmented reality microscope system enables you to overlay text and digital images over your microscope’s field of view, improving the speed and efficiency of your microscope-based manufacturing tasks | Blog Post3 Ways Augmented Reality Microscopes Speed Up Manufacturing Tasks
|
SZX-AR1Introduction Video |