Olympus Vanta™ portable X-ray fluorescence (pXRF) analyzers provide real-time data of geologic samples containing rare-earth elements (REEs). These seventeen elements are crucial to the green energy revolution and production in nearly every industry, prompting a need to increase domestic production of these metals.1 As a result, there is market value for identifying these materials in real time. Vanta pXRF analyzers have superb limits of detection and a high number of quantifiable REEs, enabling effective exploration and identification of REEs in the field.
The search for deposits with high-value REEs is desirable to the mining and exploration industry as these materials are used in a wide range of products, including green technology, consumer electronics, medical imaging machines, and defense weaponry. Recent economic changes have prompted many regions to find and use domestic REE deposits and process these ores at home.2
The seventeen REEs are commonly found and grouped with thorium (Th) and uranium (U). The Olympus pXRF software includes the near REEs lanthanum (La), cerium (Ce), praseodymium (Pr), neodymium (Nd), as well as scandium (Sc), yttrium (Y), thorium (Th), uranium (U), caesium (Cs), and barium (Ba).
pXRF Performance on REEs and Associated Elements
The graphs below illustrate the out-of-the-box performance of Vanta pXRF analyzers on a range of certified reference materials (CRMs) in various kits supplied by Ore Research and Exploration Assay Standards (OREAS). Exceptional accuracy and precision between the CRM data and the calculated concentrations from the Vanta pXRF analyzers show how Vanta analyzers can provide excellent, high-quality data on REE-containing ores and deposits.
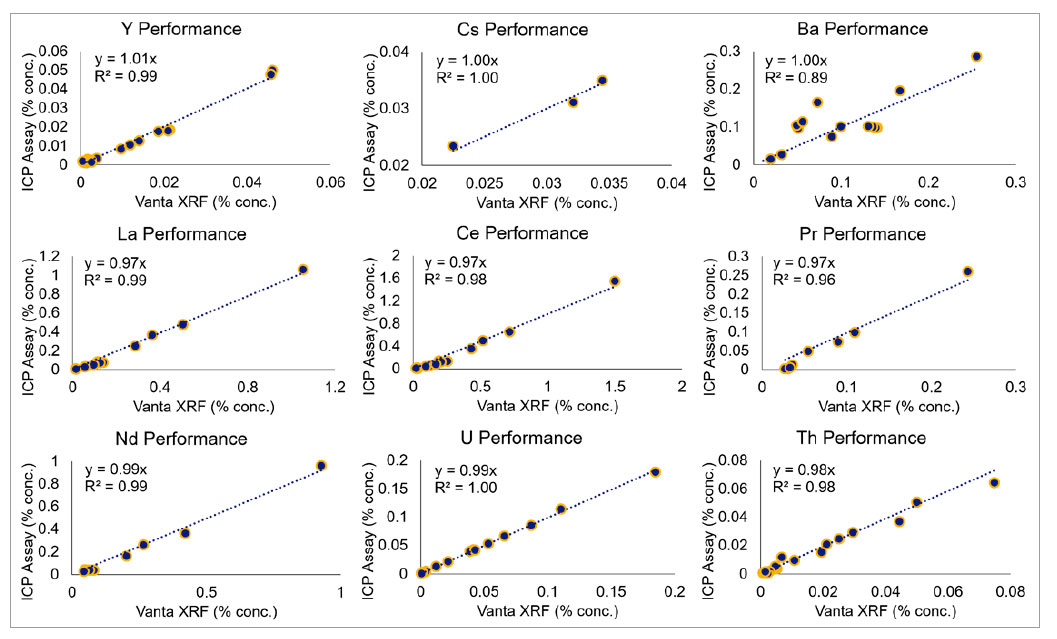 Figure 1. Vanta pXRF performance of rare-earth elements and associated elements compared to standards using various kits supplied by OREAS.
Figure 1. Vanta pXRF performance of rare-earth elements and associated elements compared to standards using various kits supplied by OREAS.
The Vanta pXRF analyzer demonstrated high performance for a variety of ore samples, including fluorocarbonates and secondary phosphates. The fluorocarbonates are generally bastnasite, a provider of yttrium, cerium, and lanthanum, while the phosphates are usually monazite, which contain lanthanum, cerium, praseodymium, and neodymium, along with the heaver REEs samarium and gadolinium and the weakly radioactive thorium. As shown by the graphs at each level, the Vanta performs accurately and precisely across both the bastnasite and the monazite, as indicated by the elements’ slopes and the R2 being close to 1.
Both REE-containing minerals can be found in similar forms worldwide. Various mines and deposits, including the world-famous Bayan Obo mine, the Mountain Pass mine, Lemhi Pass, and Elk Creek, contain similar geologies and mineralization to the tested standards.3 The Mountain Pass mine in California, for example, contains gneisses, a specific type of metamorphic rock, of a similar age to several OREAS samples.
Much data is also publicly available that shows how the Vanta pXRF analyzers can produce excellent quality data on partially prepared and unprepared samples. View some of this data here:
- Handheld XRF for Soil Surveys: Geochemistry of Rock Outcrops, Soils, and Sediments
- Handheld XRF in Exploration Drilling: Reverse Circulation/Rotary Air Blast and Diamond Core
- Portable XRF for Gold (Au) and Au Pathfinders for Mineral Exploration and Ore Body Vectoring
REE Mining and Processing
While many countries have not mined REEs domestically in decades, there has been a recent push to mine and process these valuable materials. Multiple mining facilities and processing plants, such as those at the Mountain Pass mine in eastern California, Round Top mine in West Texas, the Elk Creek mine in Nebraska,the White Mesa Mill in Utah, and the Bear Lodge mines in Wyoming, are home to high-quality deposits of REEs.3 While there are 160 known minerals that contain rare earths, four are primarily mined for this purpose: bastnaesite, laterite clays, monazite, and loparite. Other minerals such as apatite, gadolinite, and xenotime are sometimes used.
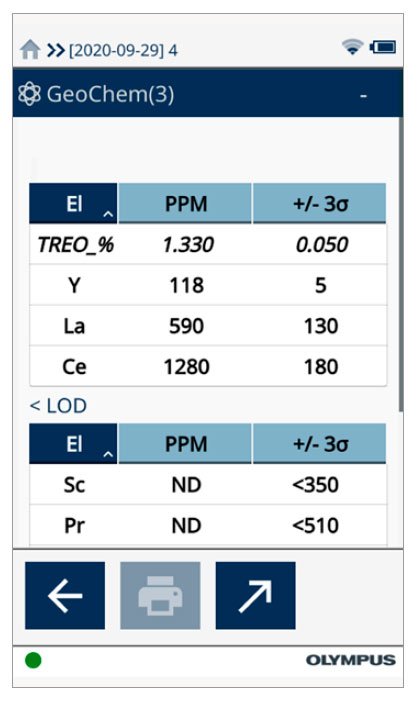
While rare-earth elements are not technically “rare,” they are not typically found in large concentrations or veins like gold (Au) or other minerals. This makes it difficult to identify and mine these materials. Also, processing of rare-earth ores is a unique and largely chemical engineering task, far more complex than processing Au and base metal ores. The Vanta pXRF analyzer can detect down to double-digit ppm levels of REE, making it the ideal tool for fast, on-site identification of these valuable elements. Further, the Vanta analyzer can calculate the concentration of rare-earth oxides in the field, as shown below. Figure 2.
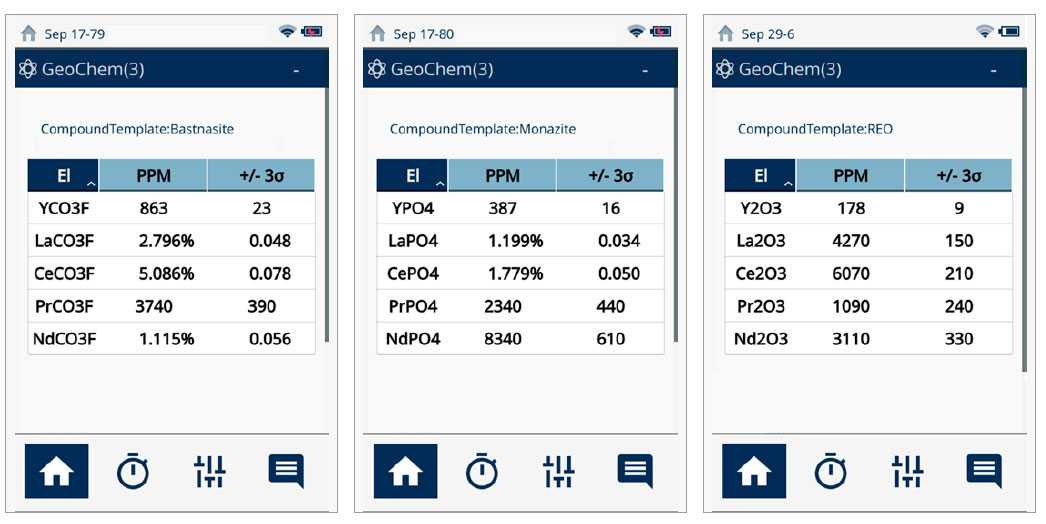 Figure 2. The Vanta pXRF analyzer calculates compound concentration of common REE-containing minerals.
Figure 2. The Vanta pXRF analyzer calculates compound concentration of common REE-containing minerals.
While identification in polymetallic ore can be difficult, processing these materials can present extra challenges. Removing and purifying the rare earths from their native ore (less than 10% combined rare-earth ore) into useable material (greater than 60% rare-earth ore) can usually be done by magnetic, electrostatic, or gravimetric processes. From here, various non-rare earth ores are dissolved with acid or heated away, leaving a combination of rare-earth ores that can be extracted in their metallic state by other chemical processes. Portable XRF can be used at each stage of this purification process to quantify the rare-earth ores or earth-abundant compounds that are removed during the process, such as calcite, silica, or magnetite. These processes, many of which were developed by the US Atomic Energy Commission after World War II, can produce REEs above 99.9% purity.4, 5
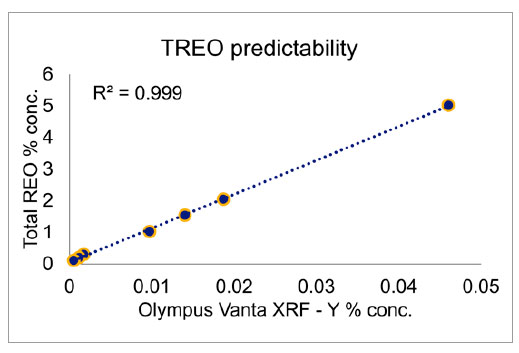
Yttrium as a Rare-Earth Oxide Pathfinder
Because complete analysis of all seventeen REEs is either impossible (in the case of pXRF) or prohibitively slow and expensive (in the case of ICP), it is useful to find methods of determining the total rare-earth concentration in the field in real time. Similar to how a variety of select elements, such as arsenic, copper, lead, and zinc, can be used as a pathfinder to detect gold, yttrium (Y) can be used to accomplish an analogous goal with rare-earth oxides (REO), provided the target REE mineral has Y in a detectable level in its composition. Using the CRMs, the yttrium concentration can be compared to the total rare-earth oxide (TREO) concentration. When plotted with the Y concentration on the horizontal axis and the TREO concentration on the vertical axis, the Y concentration is shown to be a great predictor of the TREO concentration. A similar relationship has been seen in testing similar samples from active REE projects, such as the Lofdal Heavy Rare-Earths Project in northern Namibia, where the dominant rare-earth mineral is xenotime (YPO4), which is enriched in many of the heavy rare-earth earths. Using the PseudoElements function on the Olympus Vanta pXRF analyzer, the TREO concentration can be calculated in real time in the field. All calculations can be done on the instrument during a test, as shown as shown in Figure 4. Recycling REEs with pXRF AnalysisBecause rare-earth elements are expensive, recycling their metals from consumer goods and other technologies, such as catalytic converters, has become increasingly important.6 However, due to the high complexity of current electronics, it has become difficult to do at costs that make it viable. For example, modern cell phones contain up to 65 elements, making traditional recycling techniques difficult. Fortunately, the Vanta analyzer can scan a full suite of elements from magnesium (Mg) through uranium (U), including the rare earths, to make it easier to identify and recycle rare earths from electronics, cars, and other consumer and industrial goods. Recycling REEs enables them to be repurposed for various uses, such magnets for green technology and consumer goods.7 |
|
References
- Schulz, K.J., DeYoung, J.H., Seal, R.R. and Bradley, D.C. eds., 2018. Critical Mineral Resources of the United States: Economic and Environmental Geology and Prospects for Future Supply. Geological Survey.
- Long, K.R., Van Gosen, B.S., Foley, N.K. and Cordier, D., 2012. The principal rare earth elements deposits of the United States: A summary of domestic deposits and a global perspective. In Non-Renewable Resource Issues (pp. 131-155). Springer, Dordrecht.
- Van Gosen, B.S., Verplanck, P.L. and Emsbo, P., 2019. Rare earth element mineral deposits in the United States (No. 1454). US Geological Survey.
- Frank H. Spedding, Harley A. Wilhelm, Wayne H. Keller, Donald H. Ahmann, Adrian H. Daane, Clifford C. Hach, and Robert P. Ericson. Industrial Engineering Chemistry 1952 44 (3), 553-556
- Spedding, F.H., 1949. Large-scale separation of rare-earth salts and the preparation of the pure metals. Discussions of the Faraday Society, 7, pp.214-231.
- Bleiwas, D.I., 2013. Potential for recovery of cerium contained in automotive catalytic converters.
- Goonan, T.G., 2011. Rare earth elements: End use and recyclability (p. 15). Reston: US Department of the Interior, US Geological Survey.